Search Results
Showing results 1 to 20 of 23

Dye Detective
Source Institutions
Learners analyze mixtures of dyes using filter paper chromatography. They place spots of the different dyes at the bottom of a piece of filter paper, and hang the paper to touch the surface of water.
It's A Gas!
Source Institutions
Visitors mix water and sodium bicarbonate (baking soda) in a large flask. They then add citric acid to the mixture and stopper the flask. The resulting reaction creates carbon dioxide gas.

Cartesian Diver
Source Institutions
In this demonstration, learners observe the effects of density and pressure. A "diver" constructed out of a piece of straw and Blu-Tack will bob inside a bottle filled with water.

Hot and Cold
Source Institutions
In this activity, learners explore temperature changes from chemical reactions by mixing urea with water in one flask and mixing calcium chloride with water in another flask.
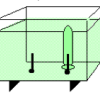
Electrolysis
Source Institutions
Using electrolysis, learners produce hydrogen gas and oxygen gas from water molecules in a solution.
All Mixed Up!: Separating Mixtures
Source Institutions
Visitors separate a mixture of pebbles, salt crystals, and wood shavings by adding water and pouring the mixture through a strainer.
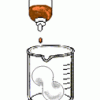
Foam Peanuts
Source Institutions
Learners compare the properties and solubilities of Styrofoam (TM), ecofoam packing peanuts, and popcorn. First, the solubility of each substance is tested in water.
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.

Forwards and Backwards
Source Institutions
In this activity, learners explore acids and bases by preparing six solutions that combine vinegar and ammonia, ranging from acid (all vinegar) to base (all ammonia).

All Mixed Up!
Source Institutions
In this activity, learners separate a mixture of pebbles, salt crystals, and wood pieces. They add water and pour the mixture through a strainer.
Egg Osmosis
Source Institutions
Visitors observe three beakers. One beaker contains an egg immersed in vinegar. Visitors observe carbon dioxide gas escaping from the shell as the calcium carbonate reacts with the vinegar.
Forwards and Backwards: pH and Indicators
Source Institutions
Visitors prepare six solutions combining vinegar and ammonia that range incrementally from acid (all vinegar) to base (all ammonia).

It's a Gas!
Source Institutions
In this activity, learners explore two properties of gases: gases take up space and exert pressure. Learners assemble two flasks and a beaker, connecting them with stoppers and tubing.

Rocket Science
Source Institutions
Learners create a small explosion by collecting hydrogen and oxygen gas together and squeezing them into a flame.

Soil Density
Source Institutions
In this activity, learners will test soil content using their sample, some water and a container that seals.

Waterproof Hanky
Source Institutions
In this physics demonstration, learners will be surprised when a handkerchief holds water in an upside-down glass.
Yeast Balloons
Source Institutions
Visitors observe a bottle with a balloon attached around the mouth. The bottle contains a solution of yeast, sugar, and water.
Currently Working: Testing Conductivity
Source Institutions
Visitors test solutions of water, sugar, salt, and hydrochloric acid and the solids salt and sugar. They clip leads from the hand generator to wires immersed in each substance.
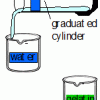
Natural Buffers
Source Institutions
Learners use a universal indicator to test the amount of sodium hydroxide needed to change the pH of plain water compared with the amount needed to change the pH of gelatin.

Electrolysis
Source Institutions
Learners observe two joined glass tubes containing a conductive salt solution. Electrodes are passing an electric current through the water.
