Search Results
Showing results 1 to 20 of 34

Water Treatment
Source Institutions
Water treatment on a large scale enables the supply of clean drinking water to communities.

Water Body Salinities II
Source Institutions
In this activity, learners discuss the different salinities of oceans, rivers and estuaries.
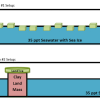
Causes and Effects of Melting Ice
Source Institutions
In this activity, learners explore the concept of density-driven currents (thermohaline circulation) and how these currents are affected by climate change.

Water Body Salinities I
Source Institutions
In this activity, learners investigate the different salinity levels of oceans, rivers and estuaries.

Exploring Forces: Gravity
Source Institutions
In this nanoscience activity, learners discover that it's easy to pour water out of a regular-sized cup, but not out of a miniature cup.
It's A Gas!
Source Institutions
Visitors mix water and sodium bicarbonate (baking soda) in a large flask. They then add citric acid to the mixture and stopper the flask. The resulting reaction creates carbon dioxide gas.

Be A Pasta Food Scientist
Source Institutions
In this activity, learners of all ages can become food scientists by experimenting with flour and water to make basic pasta.

Mystery Sand
Source Institutions
In this activity, learners play with surprising sand that doesn’t get wet! Learners explore how water behaves differently when it comes in contact with "magic sand" and regular sand.

Laser Projection Microscope
Source Institutions
In this activity, learners use a laser pointer to project a microscopic image of a liquid sample suspended from the tip of a syringe.

Cartesian Diver
Source Institutions
In this demonstration, learners observe the effects of density and pressure. A "diver" constructed out of a piece of straw and Blu-Tack will bob inside a bottle filled with water.
All Mixed Up!: Separating Mixtures
Source Institutions
Visitors separate a mixture of pebbles, salt crystals, and wood shavings by adding water and pouring the mixture through a strainer.

Forgotten Genius
Source Institutions
This series of chemistry stations is designed to accompany the PBS documentary about African-American chemist "Percy Julian: Forgotten Genius." Each of the six stations features either a chemical or p
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.

Forwards and Backwards
Source Institutions
In this activity, learners explore acids and bases by preparing six solutions that combine vinegar and ammonia, ranging from acid (all vinegar) to base (all ammonia).

Lotus Leaf Effect
Source Institutions
This is a demonstration about how nature inspires nanotechnology. It is easily adapted into a hands-on activity for an individual or groups.
Forwards and Backwards: pH and Indicators
Source Institutions
Visitors prepare six solutions combining vinegar and ammonia that range incrementally from acid (all vinegar) to base (all ammonia).

Ice Balloons
Source Institutions
In this activity, learners will explore globes of frozen water to learn how to ask and then answer 'investigable' questions. The activity web page includes a short video demonstration.

Waterproof Hanky
Source Institutions
In this physics demonstration, learners will be surprised when a handkerchief holds water in an upside-down glass.

Gravity Fail
Source Institutions
In this activity, learners try pouring water out of a regular cup and a miniature cup. It’s harder than it sounds! Learners discover that different forces dominate at different size scales.

Ready, Set, Fizz!
Source Institutions
In this activity, learners explore the chemical reaction between water and effervescent antacid tablets. This hands-on activity models how a material can act differently when it's nanometer-sized.
