Search Results
Showing results 1 to 20 of 21

Hot Stuff!: Investigation #4
Learners test two jars containing soil, one covered and one open, for changes in temperature. After placing the jars in the Sun, learners discover that the covered jar cools down more slowly.

Hot Stuff!: Investigation #1
Learners test two jars, one containing plain air and one containing carbon dioxide gas, to see their reactions to temperature changes.

Shell Shifts
Source Institutions
Ocean acidification is a big issue due to the amount of carbon dioxide humans release. CO2 in the atmosphere is absorbed into the ocean thus changing its acidity.

What's In Your Breath?
Source Institutions
In this activity, learners test to see if carbon dioxide is present in the air we breathe in and out by using a detector made from red cabbage.
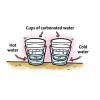
Temperature Affects the Solubility of Gases
Source Institutions
In this activity, learners heat and cool carbonated water to find out whether temperature has an effect on how fast the dissolved gas leaves carbonated water.

Build a Rocket - and a Launch Pad!
Source Institutions
In this activity, learners construct a rocket powered by the pressure generated from an effervescing antacid tablet reacting with water, and build a launch pad for their rocket.

A Mole of Gas
Source Institutions
In this two-part activity, learners use everyday materials to visualize one mole of gas or 22.4 liters of gas. The first activity involves sublimating dry ice in large garbage bag.

Film Canister Rocket
Source Institutions
In this activity, learners construct and launch rockets using simple materials and their understanding of chemical reactions.
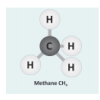
Let's Make Molecules
Source Institutions
In this activity, learners use gumdrops and toothpicks to model the composition and molecular structure of three greenhouse gases: carbon dioxide (CO2), water vapor (H2O) and methane (CH4).

Hot Stuff!: Investigation #2
Learners test two jars containing hot water, one covered with plastic and one open, for changes in temperature.

Liquid Lava Layers
Source Institutions
In this activity, learners explore the concepts of density and basic chemical reactions as they create a homemade lava lamp effect using water, oil, food coloring, and Alka-Seltzer tablets.
The Carbon Cycle: Carbon Tracker
Source Institutions
In this activity, learners play NOAA's Carbon Tracker game and discover ways to keep track of carbon dioxide and other greenhouse gases in the world.
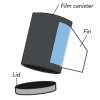
Pop Rockets
Source Institutions
In this activity, learners make film canister rocket ships. A fin pattern is glued onto the outside of the canister, and fuel (water and half an antacid tablet) is mixed inside the canister.

Breathing Blue
Source Institutions
In this activity, learners test exhaled breath for carbon dioxide and learn how to use an indicator as a simple way to measure pH.

Volcano Baseball
Source Institutions
In this game, learners are volcanoes that must complete several steps to erupt. Starting at home plate, learners draw cards until they have enough points to move to first base.

Making Naked Eggs: Eggs Without Shells
Source Institutions
This is an activity about acid-base reactions using eggs and vinegar. Learners place eggs inside a container of vinegar and leave to soak overnight.

Bubble Suspension
Source Institutions
In this activity, learners observe as soap bubbles float on a cushion of carbon dioxide gas. Learners blow bubbles into an aquarium filled with a slab of dry ice.

Hot Stuff!: Investigation #3
Learners test two jars of ice water, one covered and one open, for changes in temperature. After placing the jars in the sun, learners discover that the covered jar cools down more slowly.

Solving Dissolving
Source Institutions
The Sacred Cenote at Chichén Itzá is a sink hole, or well, containing groundwater. In this activity, learners create their own cenote using chalk, limestone, acids, and rain water.

"Boyle-ing" Water
Source Institutions
In this activity, learners explore Boyle's Law and discover that water will boil at room temperature if its pressure is lowered.
