Search Results
Showing results 1 to 11 of 11

Electroplating
Source Institutions
In this electrochemistry activity, learners will explore two examples of electroplating.

Chemical Change
Source Institutions
In this chemistry activity, learners explore the amount of copper in a new penny. Learners use toilet bowl cleaner to hollow out the interior of a penny with zinc inside.
Shocking Fruit
Source Institutions
In this activity, learners discover how a piece of fruit can act as an electrolyte, conducting electricity between two different metals.

Harvesting Chemicals from a Battery
Source Institutions
In this activity, learners take apart a used zinc-carbon dry cell battery.

Science of Sunblock
Source Institutions
This is an activity (located on page 3 of the PDF under Stained Glass Activity) about nanotechnology making its way into everyday products, such as sunscreen, and how effective these invisible particl

Build A Battery
Source Institutions
The Let's Do Chemistry "Build a Battery" activity lets participants learn how batteries work and how materials behave, change, and interact by building their own simple battery out of metal and felt w
Physical Change
Source Institutions
In this activity, learners use heat to separate zinc and copper in a penny. This experiment demonstrates physical properties and how physical change (phase change) can be used to separate matter.
Properties of Metals
Source Institutions
In this activity, learners explore the properties of metals at four stations. The stations include A) Magnetism and Breakfast Cereal; B) Conductivity of Metals; C) Alloys; and D) Metal Plating.

Penny Battery
Source Institutions
In this activity, learners light an LED with five cents. Learners use two different metals and some sour, salty water to create a cheap battery.
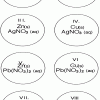
Single Replacement Micro-Reactions
Source Institutions
In this chemistry activity, learners use common chemicals and metals to explore single replacement reactions.

What's in a Penny?
Source Institutions
In this chemistry activity, learners use chemical reactions to observe the composition of an alloy.
