Source Institutions
Source Institutions
Add to list Go to activity
Activity link broken? See if it's at the internet archive
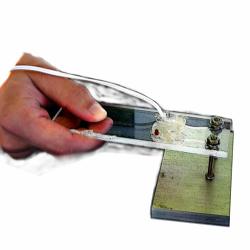
Learners test solutions of water, sugar, salt, and hydrochloric acid for electrical conductivity. They immerse leads from a lighting device (a battery pack connected to an LED) into each solution. If the solution conducts electricity, the light turns on. Learners discover that only certain substances (those with dissolved ions) conduct electricity. Original operating guide (using a fan instead of a light, and a hand-cranked generator instead of a battery) with original title "Just Charge It" is included. This activity is currently used in the Nature of Matter Unit in OMSI's Chemistry Lab. Cost estimates are per 100 learners. For safety reasons, this activity should be conducted as a demonstration for younger learners.
- 30 to 45 minutes
- Under 5 minutes
- $1 - $5 per group of students
- Ages 14 - adult
- Activity, Demonstration, Experiment/Lab Activity
- English
Quick Guide
Materials List (per group of students)
- culture plate, 4 wells
- 4 ft copper wire, 10 or 12 gauge
- 6-volt lantern battery, or 4 D-cell batteries
- LED, red preferred
- copper strip
- (3) 250-mL squeeze bottles
- 1T measuring spoon
- 1kg sodium sulfate (Na2SO4)
- 1kg sugar (C12H22O11)
- 125-mL dropper bottle
- 100mL 0.5 M hydrochloric acid (HCl)
- super glue
- hot-glue gun
- small DC motor, fan propeller attached (optional)
Subjects
-
Physical Sciences
-
Electricity and Magnetism
- Electric Circuits
-
Chemistry
- Solutions
-
States of Matter
- Liquids
-
Electricity and Magnetism
-
The Nature of Science
-
The Scientific Process
- Conducting Investigations
-
The Scientific Process
Audience
To use this activity, learners need to:
- see
- see color
- read
- touch
Learning styles supported:
- Involves hands-on or lab activities
Other
This resource is part of:
Access Rights:
- Free access
By:
Rights:
- All rights reserved, Oregon Museum of Science and Industry, 1997
Funding Source:
- National Science Foundation, 9355628
