Source Institutions
Source Institutions
Add to list Go to activity
Activity link broken? See if it's at the internet archive
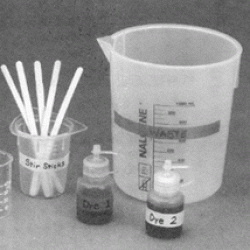
Learners add two dyes to mineral oil and water, and then compare their miscibility (how well they mix) in each. Learners discover that the molecular structure of a dye determines whether it will mix well with oil-like substances or with water-like substances. The red dye in the experiment is an industrial dye used to color wax ceramic molds. Because mineral oil has a very similar molecular structure to wax, the red dye mixes well with mineral oil. The blue dye is an industrial food coloring that mixes well with water. Most food contains water, so the blue dye works well for food coloring.
- 10 to 30 minutes
- 5 to 10 minutes
- $1 - $5 per group of students
- Ages 8 - 18
- Activity, Experiment/Lab Activity
- English
Quick Guide
Materials List (per group of students)
- Two 50-ml beakers with graduations
- Two 30-ml dropper bottles
- Two 250-ml squeeze bottles
- Wooden stirring sticks (keep four on hand)
- One 1000-ml plastic beaker
- Two 500-ml plastic bottles
- Mineral oil (keep 500 ml on hand)
- Blue food coloring (keep 25 ml on hand)
- Sudan IV indicator (keep 50 g on hand)
- One 1-gal plastic container (an empty mineral-oil container works well)
- One 100-ml plastic beaker
- One separatory funnel and stand (you can use the setup from “Making Waves,” Unit 6)
Subjects
-
Physical Sciences
-
Chemistry
- Solutions
-
States of Matter
- Liquids
-
Structure and Properties of Matter
- Atomic Structure
-
Chemistry
Audience
To use this activity, learners need to:
- see
- see color
- touch
Learning styles supported:
- Involves hands-on or lab activities
Other
This resource is part of:
Access Rights:
- Free access
By:
Rights:
- All rights reserved, Oregon Museum of Science and Industry, 1997
Funding Source:
- National Science Foundation
