Source Institutions
Source Institutions
Add to list Go to activity
Activity link broken? See if it's at the internet archive
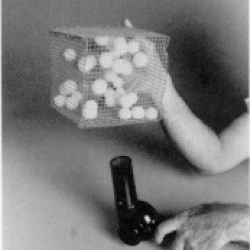
This highly visual model demonstrates the atomic theory of matter which states that a gas is made up of tiny particles of atoms that are in constant motion, smashing into each other. Balls, representing molecules, move within a cage container to simulate this phenomenon. A hair dryer provides the heat to simulate the heating and cooling of gas: the faster the balls are moving, the hotter the gas. Learners observe how the balls move at a slower rate at lower "temperatures."
- 10 to 30 minutes
- 10 to 30 minutes
- $10 - $20 per group of students
- Ages 8 - 14
- Activity, Demonstration, Model
- English
Quick Guide
Materials List (per group of students)
- 12 or more Styrofoam balls, approximately 1-1/4 inches (3 cm) in diameter (available in craft or fabric stores), or table tennis balls
- A paintbrush
- Latex paint
- A small rodent cage with wire mesh on all sides (Two plastic strawberry baskets or utility baskets with open grid sides may also be put together to form a cage.)
- Short pieces of wire or twist ties
- A hair dryer, fan, or other blower
Subjects
-
Mathematics
-
Measurement
- Rate
-
Measurement
-
Physical Sciences
-
Heat and Thermodynamics
- Heat and Temperature
- Thermodynamics and Entropy
-
Energy
- Potential and Kinetic Energy
-
States of Matter
- Gases
-
Structure and Properties of Matter
- Elementary Particles and Nuclear Physics
-
Heat and Thermodynamics
-
The Nature of Science
-
The Scientific Process
- Conducting Investigations
-
The Scientific Process
Audience
To use this activity, learners need to:
- see
- see color
Learning styles supported:
- Involves hands-on or lab activities
Other
This resource is part of:
Access Rights:
- Free access
By:
Rights:
- All rights reserved, The Exploratorium,
Funding Sources:
- National Science Foundation
- California Department of Education
- NEC Foundation of America
