Search Results
Showing results 281 to 300 of 381
Soil Secrets
Source Institutions
In this activity (located at the bottom of the page), learners investigate soil and explore the creatures that live in it.

Sink It
Source Institutions
Learners classify a variety of objects by their characteristics. They then design an experiment to determine which objects float or sink in water and add this characteristic to their classification.
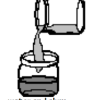
Cloudy Globs: Can You Make a White Gel From Two Clear Liquids?
Source Institutions
Using household materials, learners can make white gooey globs from clear solutions. Alum, dissolved in water, reacts with the hydroxide in ammonia to create aluminum hydroxide.

Float Your Boat
Source Institutions
In this physics activity, learners will explore buoyancy.

The Pressure's On
Source Institutions
In this chemistry activity, learners explore chemical reactions and their effects, including the kind of reaction in the human body that makes people burp!

Why is the Sky Blue?
Source Institutions
In this activity, learners use a flashlight, a glass of water, and some milk to examine why the sky is blue and sunsets are red.
Natural Buffers
Source Institutions
Learners use a universal indicator to test the amount of sodium hydroxide needed to change the pH of plain water compared with the amount needed to change the pH of gelatin.

3-2-1 POP!
Source Institutions
In this physics activity, learners build their own rockets out of film canisters and construction paper.

Wet Pennies
Source Institutions
Learners initially test to see how many drops of liquid (water, rubbing alcohol, and vegetable oil) can fit on a penny.

Hot Cans and Cold Cans
Source Institutions
Learners apply their knowledge of heat transfer to design two cans - one that will retain heat and one that will cool down quickly.

Changing Colors
Source Institutions
In this challenge, learners have to figure out in what order to combine five solutions to change the color from clear, to yellow, to blue, and back to clear.

Dissolving a Substance in Different Liquids
Source Institutions
In this activity, learners make colored sugar and add it to water, alcohol, and oil to discover some interesting differences in dissolving.

Recycling Paper
Source Institutions
In this crafty chemistry activity (on page 2 of the PDF), learners make their own paper from used paper they may have otherwise thrown away.

Feel the Heat
Source Institutions
In this design challenge activity, learners design and build a solar hot water heater. Their goal is to create a heater that yields the highest temperature change.
Milli's Super Sorting Challenge
Source Institutions
In this activity, learners separate materials based on their special properties to mimic the way recyclables are sorted at recycling centers.

Jem's Pykrete Challenge
Source Institutions
In this activity, learners make pykrete by freezing a mixture of water and a material like cotton wool, grass, hair, shredded paper, wood chips, or sawdust.

Handwashing Laboratory Activities: Fingerprint Technique
Source Institutions
In this lab (Activity #1 on page), learners compare bacteria growth on two petri dishes containing nutrient agar: one that has been touched by a finger washed only with water and one that has been tou

Yeast Balloons: Can biochemistry blow up a balloon?
Source Institutions
Using yeast, sugar, and water, learners create a chemical reaction which produces carbon dioxide (CO2) gas inside a 2-liter bottle. They use this gas to inflate a balloon.

How can Clouds Help Keep the Air Warmer?
Source Institutions
In this activity, learners explore how air warms when it condenses water vapor or makes clouds.
Cave in a Cup
Source Institutions
In this activity (page 2 of PDF under GPS: Cave Swallows Activity), learners will model how caves are formed by placing one piece of chalk in a cup of vinegar and another piece in a cup of water, then
