Search Results
Showing results 1 to 20 of 24

Best Bubbles
Source Institutions
In this activity, learners experiment with creating various types of bubble solutions and testing which ingredients form longer-lasting bubbles.

Exploring Products: Nano Sand
Source Institutions
In this activity, learners explore how water behaves differently when it comes in contact with "nano sand" and regular sand.

Cat's Meow
Source Institutions
In this chemistry activity, learners are asked to form a hypothesis about the behavior of milk as household detergents act upon it.

Snowstorm in a Jar
Source Institutions
In this activity, learners will experiment with density and chemical reactions to create a flurry activity.

How Big is Small
Source Institutions
In this classic hands-on activity, learners estimate the length of a molecule by floating a fatty acid (oleic acid) on water.

Oily Ice
Source Institutions
In this activity, learners experiment with the density of ice, water, and oil. Learners will discover that the density of a liquid determines whether it will float above or sink below another liquid.

DNA Extraction
Source Institutions
Learners use a simple process to extract DNA from strawberries.
Chemistry in the Kitchen
Source Institutions
In this kitchen chemistry activity, learners explore the chemistry of crystals by making sugar crystals, consider a common chemical reaction type responsible for the rising of muffins and cake in the

Gassy Lava Lamp
Source Institutions
In this activity, learners use oil, water, food coloring and antacid tablets to create a bubbling lava lamp. Use this activity to introduce concepts related to density, hydrophobicity vs.

Tie Dye Secret Messages
Source Institutions
In this activity, learners will write a secret message that only their friends will be able to read.

Mystery Sand
Source Institutions
In this activity, learners play with surprising sand that doesn’t get wet! Learners explore how water behaves differently when it comes in contact with "magic sand" and regular sand.

Nano Waterproofing
Source Institutions
This lesson focuses on how nanotechnology has impacted the design and engineering of many everyday items from paint to fabrics.
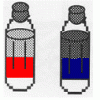
Oil and Soap
Source Institutions
Learners investigate the properties of the liquids in two bottles. One contains layers of oil and water, and one contains oil, water, and soap.

Magic Sand: Nanosurfaces
Source Institutions
This is an activity/demo in which learners are exposed to the difference bewteen hydrophobic surfaces (water repelling) and hydrophilic surfaces (water loving).
When is a Glass of Water Really Full?
Source Institutions
In this activity, learners see how many coins they can add to a full glass of water before the water overflows.
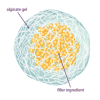
Self-Assembling Dessert Toppings
Source Institutions
This is an activity (located on page 3 of the PDF under Self-Assembly Activity) about self-assembly, the ability of molecules to assemble themselves according to certain rules.

Exploring Products: Nano Fabrics
Source Institutions
In this activity, learners explore how the application of nano-sized "whiskers" can protect clothing from stains.

Release the Grease!
Source Institutions
In this simple activity (on page 7 of the PDF), learners use water and liquid dish detergent to see which one removes lipstick better from an index card.

Floating and Falling Flows
Learners create beautiful fluid motion. They explore fluid dynamics, surface tension, solubility, and buoyancy while mixing liquids together.

Sand, Plants and Pants
Source Institutions
In this activity, learners explore how the application of nano-sized particles or coatings can change a bigger material’s properties.
